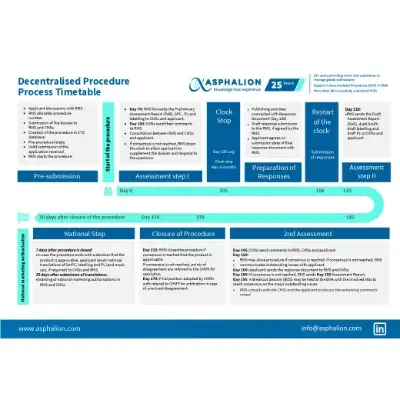
Activity

FLYER | Transforming Regulatory Submissions in Singapore
Singapore has officially begun the transition to the electronic Common Technical Document (eCTD), a major step toward a more streamlined regulatory environment in the Asia-Pacific region. Key milestones include test submissions starting now, with full acceptance of eCTD dossiers from Q2 2026. The initial launch will cover new drug applications, generic drug applications, and drug master file applications.
Download our flyer to explore the key highlights and see how Asphalion can support your transition with expert eCTD services.
If you require any assistance, you can contact us at info@asphalion.com
How to Choose Which CRM Solution Makes Sense for Your Business?
If you’re an emerging biotech or commercial-stage pharma company evaluating your CRM roadmap, this decision can shape your success across Medical, Commercial, and Patient Services functions.
Rather than asking “Which is better?”, consider asking:
-How tightly integrated is CRM with our content, regulatory, and data systems?
-Do we need a platform that supports both field teams and patient services?
-What level of flexibility do we need for omnichannel, AI, and future expansion?
-Who owns CRM administration and configuration—internal teams or managed services?
-What’s our timeline for go-live, and do we need to start simple or scale quickly?
These are the kinds of questions we help clients answer every day.
At Project Outlier, we offer an independent CRM Assessment to help life sciences teams evaluate their options clearly, with practical guidance on compliance, scalability, cost of ownership, and cross-functional needs.
Let’s talk. If you're deciding between Veeva and Salesforce, we’ll help you pick the platform that fits your roadmap.
Click here to book a free discovery call with our VP of Consulting Services, Erin Walter: http://bit.ly/3SOE1Np
Confident That Your Tech Stack Will Enable You to Scale?
How confident are you that your organization has the right technology in place to support the journey from IND submission to commercialization?
Very confident? Somewhat confident? Not confident at all?
We can help!
Download our FREE Clinical Tech Stack Blueprint to get started on building out your systems to scale all the way from IND submission to Phase 3 trials: https://lnkd.in/eYxPc6Mj

Don't Miss The Webinar!
🕒 Multiple time slots:
Americas: 6am | 10am | 2pm EDT
Europe: 12pm | 4pm | 8pm CET
⏳ Duration: 30 minutes
What To Expect:
Join us for a focused and dynamic 30-minute session where our expert speaker dives into the latest advancements in pharmacovigilance literature screening:
✓ Gain expert insights into literature screening processes
✓ Leverage AI and automation
✓ Discover practical solutions to industry pain points
✓ Learn about emerging trends shaping the future of pharmacovigilance
✓ Q&A to address your specific questions
About The Speaker:
✓ 20+ years in clinical practice & pharmacovigilance
✓ Experienced in literature screening solutions
✓ Skilled client partner
Register Now
Can't make it? Request the recording

FDA Clinical Investigator Training Course (CITC) 2024 - Promo Video
The primary goal of this clinical investigator training course is to provide participants with the essential knowledge and skills to conduct clinical trials effectively, ethically, and in accordance with regulatory standards. The course aims to prepare clinical investigators to conduct high-quality research that contributes to scientific knowledge and improves patient care.
Participants will acquire a practical understanding of:
- FDA’s approach to trial design
- Statistical issues in the analysis of trial data
- Safety concerns in the development of medical products
- Understanding preclinical information relevant to medical product development
- Clinical investigator responsibilities
Register here ➡️ FDA Clinical Investigator Training Course (CITC) 2024 - 12/10/2024 | FDA


Clinical Pharmacology Considerations for Novel Therapeutic Modalities - Webinar
This webinar will discuss the clinical pharmacology considerations for the development of novel therapeutic modalities. Our FDA subject matter experts will also address the unique considerations for oligonucleotide therapeutics and antibody-drug conjugates with respect to topics such as dose selection, exposure/response analysis, organ impairment, drug interactions, QTc assessment, and immunogenicity.
Register here ➡️ Clinical Pharmacology Considerations for Novel Therapeutic Modalities - 12/04/2024 | FDA


M13A: Bioequivalence for Immediate-Release Solid Oral Dosage Forms -- Implementing the Final Guidance - Webinar
This webinar will provide an update and overview on the final M13A Bioequivalence for Immediate-Release Solid Oral Dosage Forms guideline including major changes from the draft guideline and FDA’s current bioequivalence (BE) guidance, delineate FDA’s planning on the implementation of M13A, and address questions and provide clarifications based on questions/comments received during public consultation of the draft guideline.
Register here ➡️ M13A: Bioequivalence for Immediate-Release Solid Oral Dosage Forms -- Implementing the Final Guidance - 11/21/2024 | FDA


Clinical Pharmacology Considerations for Radiolabeled Mass Balance Studies - Webinar
This webinar will discuss the final guidance for the industry Clinical Pharmacology Considerations for Human Radiolabeled Mass Balance Studies which was published in July 2024.
Our FDA subject matter experts will also address recommendations from the guidance including, deciding whether and when to conduct the study, as well as how to design and report results from a human radiolabeled mass balance study.
Earn 1.0 CME/CPE/CNE!
Register here ➡️ Clinical Pharmacology Considerations for Radiolabeled Mass Balance Studies - 11/12/2024 | FDA


Putting the Pieces Together: REMS Logic Model in REMS Design, Implementation, and Evaluation - Webinar
This webinar aims to inform stakeholders, particularly those who design, implement and assess risk evaluation and mitigation strategies (REMS), on the recently published guidance REMS Logic Model: A Framework to Link Program Design With Assessment.
This activity is intended to provide an overview of the REMS Logic Model, including its methodological underpinnings and proposed use.
Register here ➡️ Putting the Pieces Together: REMS Logic Model in REMS Design, Implementation, and Evaluation - 11/07/2024 | FDA


FDA Clinical Investigator Training Course (CITC) 2024
The primary goal of this clinical investigator training course is to provide participants with the essential knowledge and skills to conduct clinical trials effectively, ethically, and in accordance with regulatory standards. The course aims to prepare clinical investigators to conduct high-quality research that contributes to scientific knowledge and improves patient care.
Register here ➡️ FDA Clinical Investigator Training Course (CITC) 2024 - 12/10/2024 | FDA